Aging and the Science of Longevity: What Really Works
Throughout history, humanity has aspired to extend life, searching for an elusive elixir capable of halting...
Introduction: The Quest for Longevity
Throughout history, humanity has aspired to extend life, searching for an elusive elixir capable of halting the aging process or even defying death itself. While the notion of overcoming the natural process of bodily decay has often been regarded as science fiction, rapid advancements in the study of aging are shifting this perspective, with recent breakthroughs in cellular biology deepening our understanding of the physiological processes underpinning aging. These have catalyzed the rise of a ground-breaking scientific field with the potential to slow, halt and possibly even reverse the aging process.
The science of longevity has shifted its focus from merely prolonging life to enhancing healthspan, the period of life spent in good health, free from chronic illness while advances in healthy aging research reveal that cellular aging—the decline in the functional capacity of individual cells—plays a central role in the onset of age-related diseases. By targeting the root causes of this decline, researchers are discovering proven anti-aging methods that offer not only extended years but the promise of improved quality of life as we age. Methods range from optimizing DNA repair and reducing senescent cells to evidence-based lifestyle strategies including diet, sleep hygiene and exercise. The integration of cutting-edge supplements elevates all of these interventions, facilitating actionable life extension tips that anyone can adopt.
What Causes Aging at the Cellular Level?
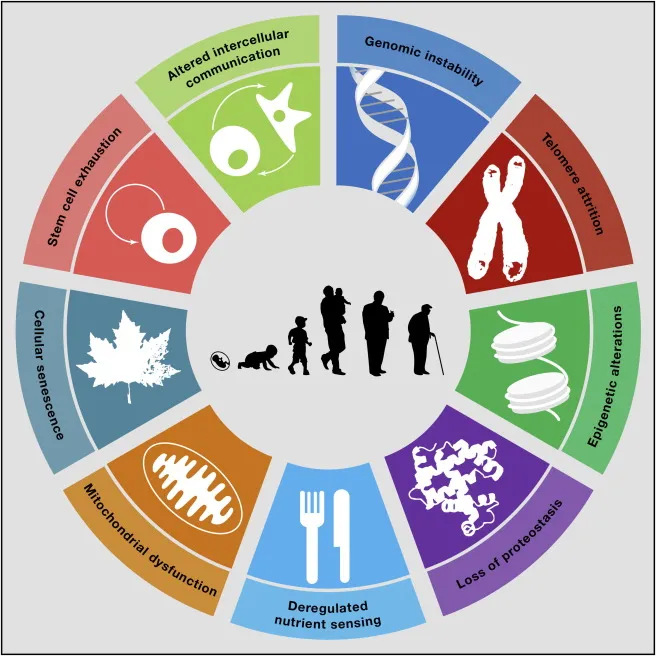
Aging is much more complex than simply physical decline. It is a highly dynamic process of physiological degradation driven by interactions between genetic, metabolic, and environmental factors. At the cellular level, aging reflects the gradual accumulation of damage that reduces the body’s capacity to repair itself - a core focus of healthy aging research. These processes are best summarized by the ‘hallmarks of aging’, a framework that highlights nine interconnected mechanisms that contribute to physical and functional decline (López-Otín et al., 2013).
Genomic Instability and DNA Damage
DNA serves as the instruction manual for all cellular functions, yet it is under constant assault from both external and internal sources. UV radiation, environmental toxins, and oxidative stress generated during metabolism all contribute to DNA damage and while the body is equipped with sophisticated DNA repair systems, their efficiency decreases with age, leading to genomic instability characterized by mutations, chromosomal rearrangements and impaired cellular function. Over time, this instability accelerates the aging process and increases the risk of chronic conditions such as cancer and cardiovascular disease (Zhao et al., 2023).

Epigenetic changes compound these effects. These are behavioral and environmental factors that result in chemical modifications which, while not altering the DNA sequence, influence how genes are expressed and these also accumulate as we age. For example, DNA methylation changes can turn off protective genes or activate harmful ones. By analyzing DNA methylation patterns in blood and other tissues, Dr. Steve Horvath developed the ‘epigenetic clock’ - an algorithm now considered the standard for biological age determination. It uses these methylation patterns as biomarkers to estimate biological age in different cells and can also be used to predict life expectancy.
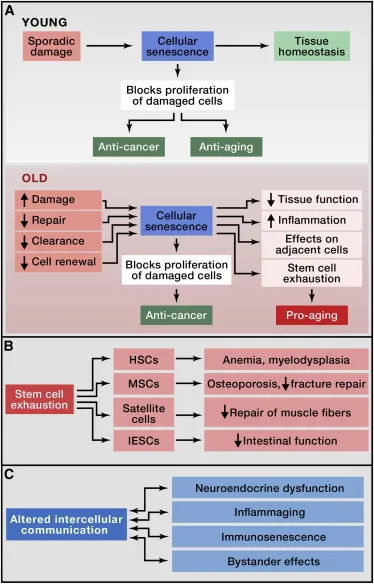
Telomere Shortening and Cellular Senescence
At the end of each chromosome is a protective cap that prevents the loss of genetic information during cell division. However, these telomeres shorten over time with each division, eventually reaching a critical length at which they can no longer divide but remain metabolically active, producing what is known as senescent cells. These cells release inflammatory molecules and proteases known as the senescence-associated secretory phenotype (SASP) and have thus become a target of proven anti-aging methods aimed at reducing tissue dysfunction and chronic inflammation. mitochondrial dysfunction and oxidative stress.


Mitochondria are the energy powerhouses of cells, producing the adenosine triphosphate (ATP), the molecular unit of currency for intracellular energy transfer and storage. They are vital to maintaining cellular health but their function declines with age, reducing energy production and increasing reactive oxygen species (ROS) levels, causing oxidative stress damage to DNA, proteins and lipids, and creating a feedback loop that exacerbates mitochondrial dysfunction. The accumulation of defective mitochondria contributes to cellular aging, reduced metabolic efficiency, and the onset of age-related diseases (Lee et al, 2020).
Chronic Inflammation
Chronic, low-grade inflammation associated with aging (inflammaging), results from the immune system's overactivation and the accumulation of senescent cells. Where a state of persistent inflammation arises, accelerated tissue degeneration occurs and is a major contributor to age-related diseases, including diabetes, cardiovascular disease, and Alzheimer’s. Longevity science therefore aims to develop interventions targeting inflammaging, through anti-inflammatory diets, supplementation and practical life extension tips to mitigate these effects and promote healthier aging (Saavedra et al 2023).
Loss of Proteostasis
Proteostasis refers to the balance of protein synthesis, folding, and degradation. With age, this equilibrium is disrupted, leading to the accumulation of damaged or misfolded proteins that impair cellular function – all hallmarks of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Enhancing autophagy (literally ‘self-eating), the cell’s natural process for clearing damaged components, is another promising avenue for restoring proteostasis and slowing age-related decline (Hartl 2017).

The Role of DNA Repair in Aging
Natural DNA repair mechanisms are essential for maintaining genomic stability and preventing the accumulation of damage that drives cellular aging. However, as we age, these systems become less effective, allowing mutations and structural abnormalities to accumulate, ultimately compromising cell function and increasing the risk of disease. Therapies that support these specialized pathways are a key target for longevity science.
Mechanisms of DNA Repair
- Base Excision Repair (BER):
Fixes oxidative damage to individual DNA bases caused by ROS.
- Nucleotide Excision Repair (NER):
Removes bulky DNA lesions caused by UV radiation or chemical exposure.
- Homologous recombination & Non-homologous end joining NHEJ (HR & NHEJ):
Repairs breaks in both strands of DNA, preventing chromosomal instability and preserving genomic integrity.
- Mismatch repair (MMR): Corrects errors of replication not detected by DNA polymerase.
Sirtuins, a family of NAD+-dependent enzymes, play a critical role in regulating these repair processes. For example, SIRT6 enhances the repair of double-strand breaks, stabilizing the genome and extending lifespan in animal studies (Korotkov et al., 2021). Often these work in concert with coenzymes like NAD+ (nicotinamide adenine dinucleotide) but their levels also decline with age, impairing repair processes and leaving cells vulnerable to damage. Supplementing with NAD+ precursors, nicotinamide mononucleotide (NMN), has shown promise in restoring NAD+ levels, enhancing DNA repair efficiency, and improving mitochondrial function (Benjamin et al., 2024).

Evidence-Based Lifestyle Changes for Longevity
Lifestyle factors profoundly influence the aging process. Decades of research into proven anti-aging methods have identified behaviors that slow biological aging, improve overall health, and reduce the risk of chronic diseases.
Nutrition and Caloric Restriction
A nutrient-rich diet is foundational to healthy aging. Caloric restriction (CR), reducing caloric intake without malnutrition, has been extensively studied and is one of the most effective interventions for extending lifespan in multiple species, activating longevity-associated pathways such as AMPK and sirtuins while suppressing mTOR, a nutrient-sensing pathway implicated in aging and chronic disease. Intermittent fasting replicates many of CR’s benefits, such as enhancing autophagy, reducing inflammation, and improving metabolic flexibility (Fontana & Partridge, 2015). The Mediterranean diet, characterized by high levels of polyphenols, healthy fats (especially omega-3 fatty acids), and lean proteins, has consistently been associated with reduced cardiovascular disease, improved cognitive function, and extended healthspan. Studies suggest that adherence to this diet reduces systemic inflammation and promotes a healthy gut microbiome, further contributing to longevity (Ross et al. 2024).


Physical Activity
Exercise is a powerful tool for promoting longevity and maintaining health. Regular aerobic activity improves cardiovascular health, enhances mitochondrial function, and reduces oxidative stress while resistance training facilitates muscle mass and bone density preservation, preventing sarcopenia (age-related muscle atrophy) and frailty commonly associated with aging (Rebelo-Marques et al., 2018). A combination of aerobic and strength-training exercises appears to offer the most benefits, with studies demonstrating that physically active individuals tend to have longer telomeres and exhibit lower levels of biomarkers associated with chronic inflammation. Engaging in just 150 minutes of moderate exercise weekly is one of the most impactful life extension tips for achieving significant improvements in lifespan and quality of life.
Stress Management and Sleep Hygiene
Chronic stress is a well-documented accelerator of aging. Elevated cortisol levels, resulting from prolonged stress, shorten telomeres, promote inflammation, and impair immune function. Effective stress management strategies, such as mindfulness meditation, yoga, and breathing exercises, have been shown to reverse some of these effects and also improve psychological resilience, which is crucial for maintaining well-being in later life (Epel et al., 2004).
Equally important is duration and quality of sleep. This critical down-time facilitates the body’s essential repair processes and deficiencies are highly correlated with negative health outcomes. During deep sleep, growth hormone levels peak, promoting tissue repair, immune function, and DNA stability, whilst disruptions to stable sleep are linked to accelerated biological aging, increased oxidative stress, and greater susceptibility to chronic disease. Prioritizing sleep hygiene by maintaining a consistent sleep schedule that allows for adequate rest and repair, combined with curating an environment conducive to quality sleep, is proven to have a profound effect on health and longevity.

How Supplements Contribute to Longevity
Supplements have emerged as hugely valuable tools in the science of longevity by addressing the biological mechanisms that underlie cellular aging and enhancing repair and defense-related biological pathways. They provide targeted support to mitigate cellular decline, reduce oxidative damage, and counteract chronic inflammation. By complementing the body’s natural processes, supplements can significantly enhance healthspan and can significantly amplify the therapeutic benefits of proper nutrition, regular physical activity, stress management, and restorative sleep.
Protecting Cellular Integrity
The restoration of NAD+ levels is a highly active and promising areas of research. NAD+ precursors nicotinamide mononucleotide (NMN) replenish declining NAD+ levels and linked to improved energy metabolism enhanced cellular resilience (Benjamin et al., 2024). Additionally, antioxidants such as vitamin C, vitamin E, and glutathione precursors combat oxidative stress by neutralizing reactive oxygen species (ROS). This process helps prevent oxidative damage to DNA, proteins, and lipids, mitigating one of the primary contributors to cellular aging.
Keeping the Lights On
Compounds such as Urolithin-A and pyrroloquinoline quinone (PQQ) are instrumental in improving mitochondrial efficiency, increasing ATP production while reducing oxidative stress. As mitochondrial function deteriorates with age, these supplements help sustain cellular energy levels and delay the metabolic slowdown associated with aging. Senolytics (NAD+-dependent enzymes) such as quercetin, fisetin, and dasatinib selectively eliminate senescent cells and in so doing, reduce the inflammatory environment associated with inflammaging, promoting healthier tissue function and overall aging (Saavedra et al., 2023). Furthermore, polyphenols like resveratrol, found in red wine, and epigallocatechin gallate (EGCG), from green tea, activate sirtuins—proteins that bolster DNA repair, stabilize the genome, and improve mitochondrial function while reducing chronic inflammation. Natural flavonoids such as quercetin and fisetin modulate sirtuin activity and protect mitochondrial function by reducing inflammatory and oxidative stress markers.
Emerging Supplement Innovations
As longevity science advances, new supplement formulations are being developed to target aging with greater precision:
Astaxanthin: A potent antioxidant derived from algae, astaxanthin has been shown to reduce oxidative stress, improve skin elasticity, and protect against UV-induced damage.
Spermidine: Found in certain foods like wheat germ and soy, spermidine enhances autophagy, the process by which cells clear damaged components. Supplementation has been linked to improved cardiovascular health and reduced markers of aging.
Alpha-Ketoglutarate (AKG): This compound supports cellular metabolism and has been shown to extend lifespan in animal studies. It may enhance energy production and reduce inflammatory markers in aging humans
The effectiveness of supplements is highly contingent on their quality and formulation. Many inexpensive supplements may not consist of quality extracts, can be of poor purity and can include fillers. Third-party testing for potency, purity, and bioavailability is essential to ensure that supplements deliver their intended benefits. Efficacious dosing is also crucial, as clinical research often defines dosages required to produce measurable effects that significantly exceed those contained in many available supplements.
The modern pursuit of longevity is no longer confined to dreams or myths—it is a tangible goal supported by cutting-edge science. By targeting the biological mechanisms of aging, healthy aging research has identified strategies to slow aging and extend both lifespan and healthspan, combining evidence-based lifestyle changes such as a nutrient-rich diet, regular exercise, and effective stress management, with high-quality supplements offering a powerful toolkit that we can all employ to optimize health as we age.
References
- Blackburn et al, (2015) Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. SCIENCE. Vol 350, Issue 6265 pp. 1193-1198
https://www.science.org/doi/abs/10.1126/science.aab3389
- Epel, E. S., et al. (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences, 101(49), 17312–17315.
https://doi.org/10.1073/pnas.0407162101 - Fontana, L., & Partridge, L. (2015). Promoting health and longevity through diet: From model organisms to humans. Cell, 161(1), 106–118.
http://dx.doi.org/10.1016/j.cell.2015.02.020
Saavedra, D., Añé-Kourí, A.L., Barzilai, N. et al. Aging and chronic inflammation: highlights from a multidisciplinary workshop. Immun Ageing 20, 25 (2023). https://doi.org/10.1186/s12979-023-00352-w
- Zhao, Y., Simon, M., Seluanov, A. et al. DNA damage and repair in age-related inflammation. Nat Rev Immunol 23, 75–89 (2023). https://doi.org/10.1038/s41577-022-00751-y
- F. Ulrich Hartl (2017). Protein Misfolding Diseases. Annual Review of Biochemistry, 86, 21-26 https://doi.org/10.1146/annurev-biochem-061516-044518
- Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115.
https://doi.org/10.1186/gb-2013-14-10-r115 - Lee, D., Jo, M. G., Kim, S. Y., Chung, C. G., & Lee, S. B. (2020). Dietary Antioxidants and the Mitochondrial Quality Control: Their Potential Roles in Parkinson's Disease Treatment. Antioxidants (Basel, Switzerland), 9(11), 1056. https://doi.org/10.3390/antiox9111056
- López-Otín, C., et al. (2013). The hallmarks of ageing. Cell, 153(6), 1194–1217.
http://dx.doi.org/10.1016/j.cell.2013.05.039 - Ross, F. C., Patangia, D., Grimaud, G., Lavelle, A., Dempsey, E. M., Ross, R. P., & Stanton, C. (2024). The interplay between diet and the gut microbiome: implications for health and disease. Nature reviews. Microbiology, 22(11), 671–686. https://doi.org/10.1038/s41579-024-01068-4
- Korotkov, A., Seluanov, A., & Gorbunova, V. (2021). Sirtuin 6: linking longevity with genome and epigenome stability. Trends in cell biology, 31(12), 994–1006. https://doi.org/10.1016/j.tcb.2021.06.009
- Rebelo-Marques, A., et al (2018) Aging Hallmarks: The Benefits of Physical Exercise. Endocrinol, Sec. Endocrinology of Aging Volume 9 – 2018. https://doi.org/10.3389/fendo.2018.00258
- Benjamin, C., & Crews, R. (2024). Nicotinamide Mononucleotide Supplementation: Understanding Metabolic Variability and Clinical Implications. Metabolites, 14(6), 341. https://doi.org/10.3390/metabo14060341
Read next



















